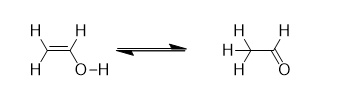
Types of Organic Reactions
Organic compounds change chemically by transferring groups to become more stable. These changes are called organic reactions. These transformations are referred to as organic reactions.
There are four main types of organic reactions: substitution, addition, elimination and rearrangement reactions.
1. Substitution Reactions
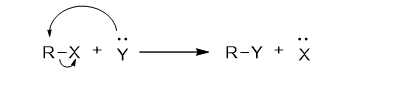
Substitution reactions, also called displacement reactions, occur when one atom or group in a molecule is replaced by another atom or group, creating new products.
The general mechanism of substitution reaction is:
There are two main types of substitution reactions, categorized by the type of reactants involved:
- Nucleophilic Substitution Reactions
- Electrophilic Substitution Reactions
Nucleophilic Substitution Reactions
In nucleophilic substitution reactions, a nucleophile (Nu), which has a lone pair of electrons, attacks the molecule and forms a new bond by replacing the leaving group (nucleofuge). The nucleophile donates a pair of electrons to form the bond, while the leaving group departs with its own pair of electrons.
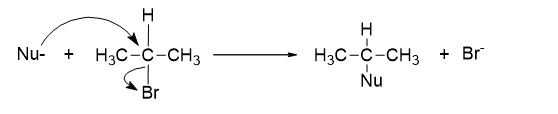
Nucleophilic substitution reactions are classified into two types based on the substrate:
- SN1 reactions
- SN2 reactions
Electrophilic Substitution Reactions
In electrophilic substitution reactions, an electrophile replaces the leaving group. An electron-deficient species (electrophile) attacks an electron-rich substrate, replacing the leaving group, which is often a hydrogen ion (H+).
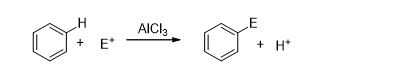
Electrophilic substitution reactions are divided into two types based on the substrate:
- Aliphatic Electrophilic Substitution Reactions
- Aromatic Electrophilic Substitution Reactions
2. Addition Reactions
Addition reactions occur in molecules with double or triple bonds. These reactions involve two or more molecules combining to form new products.
Addition reactions are categorized into two main types:
- Electrophilic Addition Reactions
- Nucleophilic Addition Reactions
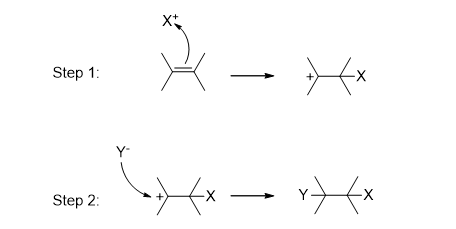
Electrophilic Addition Reactions
In electrophilic addition reactions, an electrophile (a positively charged species) approaches a double or triple bond.
In the first step, it transforms the pi bond (π) into sigma bonds (σ). In the second step, an electron-rich species attacks the positive center (usually a carbocation), resulting in the formation of an addition product.
Nucleophilic Addition Reactions
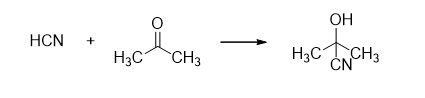
Nucleophilic addition reactions are the opposite of electrophilic addition reactions. Here, a nucleophile attacks a double or triple bond, converting the pi bond to a sigma bond.
In the first step, the electron-rich nucleophile (Nu–) attacks the double or triple bond of a carbon atom, creating a carbanion. In the second step, this carbanion combines with a positive species to form the addition product.
3. Elimination Reactions
Elimination reactions are the opposite of addition reactions. In these reactions, sigma bonds are converted into pi bonds by removing two or more atoms or groups, resulting in the formation of double or triple bonds.
Elimination reactions are of three types:
- Alpha (α) Elimination Reactions
- Beta (β) Elimination Reactions
- Gamma (γ) Elimination Reactions
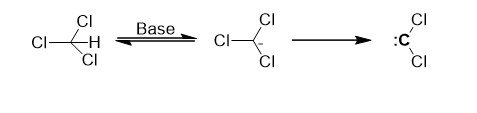
Alpha (α) Elimination Reactions
In alpha elimination reactions, both groups are removed from the same atom. This leads to the formation of carbenes in carbon compounds and nitrenes in nitrogen compounds.
Beta (β) Elimination Reactions
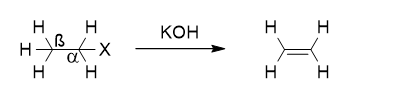
In beta elimination reactions, two groups are removed from adjacent carbon atoms. One group is removed from the alpha carbon, and the other from the beta carbon.
This is the most common type of elimination reaction. Beta elimination can occur in one step (E2) or in two steps (E1).
Beta elimination reactions are of two types:
- E1 Reaction (Unimolecular)
- E2 Reaction (Bimolecular)
Gamma (γ) Elimination Reactions
In gamma elimination reactions, a three-membered ring structure is formed. One atom is removed from the alpha carbon, and the other is removed from the gamma carbon. These three-membered rings are very unstable due to angle strain.
4. Rearrangement Reactions
Rearrangement reactions occur when an atom or group of atoms moves from one part of a molecule to another. This creates structural isomers of the original molecule.
The connections between groups change within the molecule, often involving migrations from one atom to a neighboring atom, known as 1,2 shifts. However, sometimes these migrations can cover longer distances.