Lithium-Ion Battery
A lithium-ion (Li-ion) battery is a rechargeable battery that stores energy by allowing lithium ions (Li+) to move in and out of an electrically conductive material. This type of battery is commonly used in portable electronics and electric vehicles.
For a long time, nickel-cadmium batteries were the only option for portable devices like wireless communication tools and mobile computers. In the early 1990s, nickel-metal-hydride and lithium-ion batteries started competing for customer approval. Today, lithium-ion batteries are the most rapidly advancing and promising type of battery.
History
-
Research on rechargeable Li-ion batteries began in the 1960s, with NASA creating a CuF₂/Li battery in 1965.
-
The modern Li-ion battery’s foundation was laid in 1974 by British chemist M. Stanley Whittingham, who used titanium disulfide (TiS₂) as a cathode material.
-
In 1980, Ned A. Godshall, Koichi Mizushima, and John B. Goodenough improved on this by using lithium cobalt oxide (LiCoO₂), which was more stable and offered higher voltage.
-
In the 1990s, energy density was further increased by switching to graphite anodes.
-
In 2019, John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino were awarded the Nobel Prize in Chemistry for their key roles in developing the modern lithium-ion battery.
Types of Lithium-Ion Batteries
Various types of lithium-ion batteries are present:
Lithium Cobalt Oxide
Lithium Cobalt Oxide (LiCoO₂), often called LCO, is known for its high energy density, making it a popular choice for mobile phones, laptops, and digital cameras. This battery uses a cobalt oxide cathode and a graphite carbon anode.
Lithium Manganese Oxide
Lithium Manganese Oxide (LiMn₂O₄), or LMO, was first described in 1983 and commercialized by Moli Energy in 1996. This battery uses a manganese oxide cathode and features a three-dimensional spinel structure that enhances ion flow, leading to lower internal resistance and better current handling.
Although it has about one-third less capacity than Li-cobalt, its design flexibility lets engineers optimize it for longer life, higher current, or greater capacity.
LMO batteries are preferred for power tools, medical devices, and various electric power systems.
Lithium Nickel Manganese Cobalt Oxide
Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO₂), or NMC, is a highly successful Li-ion battery system that combines nickel, manganese, and cobalt in its cathode.
The battery usually features a silicon-based anode and a cathode composed of almost equal parts nickel, manganese, and cobalt, commonly known as a 1-1-1 composition.
NMC batteries are commonly used in power tools, e-bikes, and other electric power systems.
Lithium Iron Phosphate
In 1996, researchers at the University of Texas and others discovered that using phosphate as a cathode material in lithium batteries can enhance performance. Lithium Iron Phosphate (LiFePO₄), or LFP, provides strong electrochemical performance with low resistance, due to its nano-scale phosphate material.
The main advantages of LFP batteries include high current capacity, long lifespan, good thermal stability, and improved safety and durability under stress.
Lithium Titanate
Lithium Titanate (Li₂TiO₃), or LTO, has been used in batteries since the 1980s. In these batteries, lithium titanate replaces the graphite in the anode and forms a spinel structure. The cathode can be made of lithium manganese oxide or NMC.
LTO batteries are commonly used in electric powertrains, uninterruptible power supplies (UPS), and solar-powered street lights.
Components of Li-Ion Battery
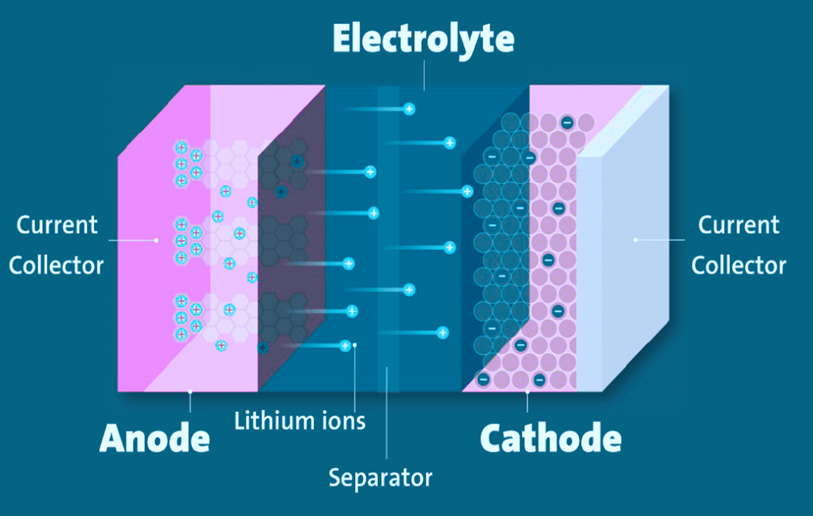
- Electrodes: The positive and negative ends of a battery cell, connected to the current collectors.
- Anode: The negative electrode.
- Cathode: The positive electrode.
- Electrolyte: A liquid or gel that allows electricity to flow.
- Current collectors: Metal foils attached to each electrode, connecting the battery to its terminals, which carry electric current between the battery, the device, and the power source.
- Separator: A porous plastic film that keeps the electrodes apart while allowing lithium ions to move between them.
Working of Li-Ion Batteries
In a lithium-ion battery, lithium ions (Li+) move between the cathode and anode inside the battery, while electrons flow in the opposite direction through the external circuit. This movement generates the electrical current that powers devices. These lithium ions are small enough to move through a micro-permeable separator that separates the anode from the cathode.
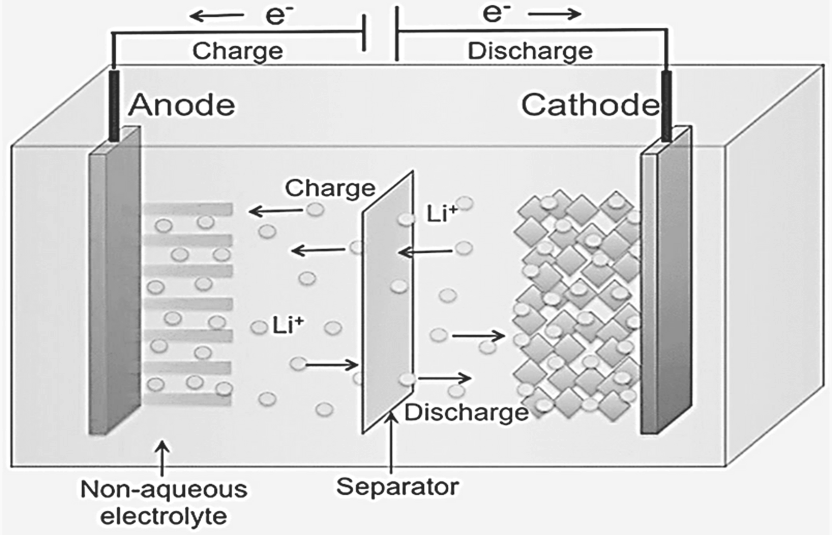
Charging
When the battery is charging, lithium ions (Li+) leave the cathode (positive electrode) and move to the anode (negative electrode) for storage.
Discharging
During discharge, lithium atoms in the anode lose electrons, becoming ions that travel through the electrolyte to the cathode, where they reunite with their electrons and become neutral again.
Characteristics of Lithium-Ion Batteries
Lithium-Ion batteries have both advantages and disadvantages that are important to consider for their use in specific situations.
Advantages
-
High energy density with the potential for even greater capacity.
-
No need for extended priming when new; just one regular charge is enough.
-
Low self-discharge rate, less than half of what nickel-based batteries experience.
-
Low maintenance, with no need for periodic discharge or concerns about memory effect.
-
Specialized cells can deliver very high currents, ideal for power tools.
Disadvantages
-
Requires a protection circuit to keep voltage and current within safe limits.
-
Prone to aging, even when not in use; storing at 40% charge in a cool place can slow this process.
-
Shipping restrictions may apply for larger quantities, though personal carry-on batteries are exempt.
-
Costly to produce, about 40% more expensive than nickel-cadmium.
-
Still evolving, as the materials and chemicals used continue to change.
Applications of Lithium-Ion Batteries
Lithium-ion batteries are used in:
-
Mobile phones
-
Laptops and tablets
-
Wearable technology such as wireless headphones and smartwatches.
-
Electric vehicles, including cars, buses, trains, bikes, scooters, and hoverboards
-
Portable and stationary power banks
-
Renewable energy storage
-
Medical devices that are implanted
-
Cordless power tools
-
Drones
-
Satellites
Hey people!!!!!
Good mood and good luck to everyone!!!!!