Nickel-Cadmium Battery
The nickel-cadmium battery (Ni-Cd or NiCad) is a rechargeable battery that uses nickel oxide hydroxide and metallic cadmium for its electrodes. The name Ni-Cd comes from the chemical symbols for nickel (Ni) and cadmium (Cd). While “NiCad” is a trademark of the SAFT Corporation, people often use it to refer to all Ni-Cd batteries.
Nickel-cadmium (Ni-Cd) batteries are an important part of the history of rechargeable batteries. They were among the first types of rechargeable batteries used in many everyday items. Ni-Cd batteries have qualities that make them useful in specific situations, and they’ve been popular for certain uses since they first became available for purchase.

History
The history of Nickel-Cadmium (Ni-Cd) batteries began in 1899 when Swedish inventor Waldemar Jungner created them. Despite facing challenges at first, Jungner’s invention paved the way for better rechargeable batteries. Ni-Cd batteries became popular because they were tougher chemically and could store more energy than lead-acid batteries.
Jungner’s company, Ackumulator Aktiebolaget Jungner, later became Saft AB, a big maker of Ni-Cd batteries. In the 1930s and 1940s, improvements like coated electrodes and sealed designs made these batteries even better.
Ni-Cd batteries became widely used in the late 20th century. By 2000, about 1.5 billion Ni-Cd batteries were made every year. They powered things like portable gadgets, tools, and flashlights.
Working of Ni-Cd Batteries
Ni-Cd batteries work using reversible chemical reactions with three main parts:
- A nickel oxide hydroxide cathode
- A cadmium-based anode
- An alkaline electrolyte, usually potassium hydroxide
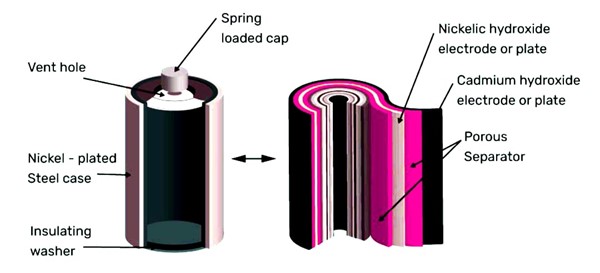
Ni-Cd batteries are typically encased in metal with a sealing plate that has a self-sealing safety valve. Inside, the positive and negative electrodes are separated by the separator and rolled into a spiral shape, called the “jelly-roll” design. This design enables Ni-Cd cells to deliver a much higher maximum current compared to similarly sized alkaline cells.
Here’s a simple explanation of the electrochemical reactions:
Discharging
When the battery discharges, the cadmium anode reacts with hydroxide ions to form cadmium hydroxide and electrons. Meanwhile, the nickel oxide hydroxide cathode is reduced to nickel hydroxide by these electrons. This process creates a flow of electrons from the anode to the cathode through an external circuit, providing electrical power.
Chemical reactions during discharge:
At the cadmium electrode:
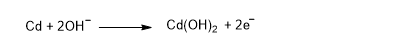
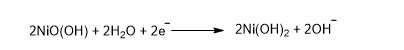
At the nickel oxide electrode:
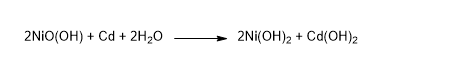
The overall discharge reaction:
Charging
When the battery charges, these reactions are reversed. An external electrical current converts nickel hydroxide back to nickel oxide hydroxide at the cathode and cadmium hydroxide back to cadmium metal at the anode. This process prepares the battery for another discharge cycle.
The alkaline electrolyte, usually potassium hydroxide, is not consumed in the reaction, so its specific gravity does not indicate the state of charge, unlike in lead-acid batteries.
Characteristics of Nickel-Cadmium Batteries
Nickel-Cadmium (Ni-Cd) batteries have both advantages and disadvantages that are important to consider for their use in specific situations.
Advantages
- High Charging/Discharging Rates: Ni-Cd batteries can handle high charging and discharging rates without losing performance due to their low internal resistance. This makes them ideal for applications needing a lot of power quickly.
- Durability and Longevity: These batteries can endure many charge and discharge cycles and can handle deep discharges. With proper care, they can last for many years, making them cost-effective.
- Performance in Extreme Temperatures: Ni-Cd batteries perform well over a wide temperature range and do not overheat easily.
- Reliable Output: They provide a consistent nominal voltage of 1.2 V per cell, which remains stable during discharge. This reliability is important for devices that need a predictable power output.
Disadvantages
- Environmental Concerns: Ni-Cd batteries contain cadmium, a toxic heavy metal that can harm the environment and health if not disposed of properly. This has led to restrictions on their sale, especially in the European Union.
- Memory Effect: Repeatedly recharging a Ni-Cd battery after only partially discharging it can cause the battery to lose its full capacity over time. This can be managed with proper charging techniques but remains an issue.
- Energy Density: Ni-Cd batteries are heavier and larger compared to newer battery technologies with the same capacity, which can be a drawback for portable devices.
- Cost and Availability: While they can be cost-effective for certain applications, the shift to other battery types has reduced the availability of Ni-Cd batteries, making them less common and less able to benefit from mass production.
Uses of Nickel-Cadmium Batteries
Ni-Cd batteries were once widely used in consumer devices before being replaced by newer technologies. Today, they are commonly used in:
Emergency Lighting and Backup Systems: Ni-Cd batteries are perfect for emergency lighting and backup power because they hold their charge well, provide stable power, can handle deep discharges, and work well in various conditions.
Aerospace and Military Applications: Their durability and ability to perform under extreme conditions make Ni-Cd batteries ideal for aerospace and military equipment, where reliability is crucial.
Power Tools: Ni-Cd batteries are still popular in cordless power tools due to their ability to deliver high surge currents, provide stable output, and withstand rough handling and extreme conditions.
Well written.
“NiCd batteries are the unsung heroes of power! 🔋 They just keep going strong, no matter what you throw at them. Total game-changers! ⚡️💥”
Thanks
Very informative
Thanks